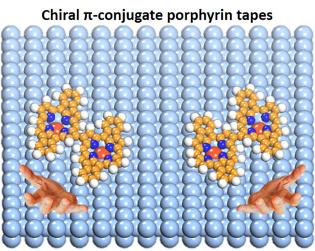
On-surface synthesis: when right meets right
Molecules cannot “decide” which partner they want to bind to. Therefore it is an enticing problem to understand how chemical reactions become selective, in our case stereoselective.
By carefully analyzing STM data and combining them with extensive DFT calculations we unraveled the reason why particular chiral molecules (i.e. they come in a “left” and “right” version) predominantly bind to molecules of the same chirality. For the case of 5,15-diphenylporphyrin (2H-DPP) on the silver (110) surface we were able to induce thermally a covalent coupling between single molecules that resulted in polymer chains with a slightly modified 2H-DPP as building block. It was surprising to see that these chains are to a large extent enantiopure, consisting of either “left” or “right” molecules.
The reason for the stereoselectivty lies in the particular molecule-surface interaction that forces the molecule into a certain rotational orientation even while diffusing on the surface at elevated temperatures. This orientation is different for the two enantiomers and hence, due to steric hindrance, a formation of racemic polymers is suppressed.